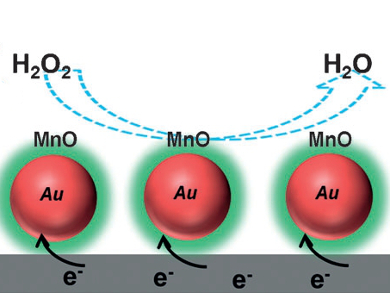
The levels of hydrogen peroxide in cells can be measured using core/shell Au/MnO nanoparticles in an electrochemical reduction reaction.
Shouheng Sun and colleagues, Brown University, Providence, RI, USA, prepared gold nanoparticles, which then reacted with a manganese complex to form a bimetallic alloy. Subsequent annealing led to a range of composites whose structures were dependent on the proportions of gold and manganese. The core/shell Au/MnO nanoparticles were then used to monitor the release of hydrogen peroxide from living cells. Electrochemical experiments showed that addition of a compound – that leads to hydrogen peroxide release – to a mixture of nanoparticles and cells results in an immediate response from the nanoparticles. The current response also varied with the type of cell: tumorigenic cells released more hydrogen peroxide and gave larger current changes than nontumorigenic cells.
This method has advantages over assays that use horseradish peroxidase, which has inherent problems such as purification and denaturing, and is more sensitive than other metal-nanoparticle-based assays.
- Core/Shell Au/MnO Nanoparticles Prepared Through Controlled Oxidation of AuMn as an Electrocatalyst for Sensitive H2O2 Detection,
Huiyuan Zhu, Aruna Sigdel, Sen Zhang, Dong Su, Zheng Xi, Qing Li, Shouheng Sun,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201406281