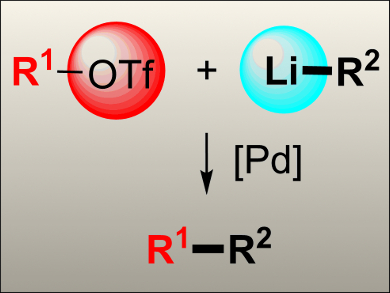
Palladium-mediated cross-coupling reactions are a particularly effective tool for C–C bond formations. Various organometallic partners are used in these reactions including organomagnesium, -tin, -zinc, -boron, -silicon, and, more recently, organolithium species. Organolithium reagents represent an attractive alternative, considering their widespread use in organic synthesis, easy preparation, versatility, and low cost. Moreover, boron, tin, zinc, or silicon reagents are frequently prepared from the corresponding lithium reagents, so the direct use of organolithium compounds would eliminate additional transformation and purification processes.
Ben L. Feringa and colleagues, University of Groningen, The Netherlands, have developed a catalytic system for the direct palladium-catalyzed cross-coupling of aryl triflates with a range of alkyl- and aryllithium reagents. This methodology is based on the use of the commercially available catalytic system [Pd2(dba)3]/DavePhos (dba = dibenzylideneacetone). The transformation features short reaction times (1–1.5 h) and affords the corresponding alkylated or arylated products in moderate to high yields.
This methodology has also been applied to the coupling of alkenyl triflates and aryllithium reagents in a straightforward synthesis of olefins by using [Pd2(dba)3]/SPhos. The direct use of organolithium reagents in these reactions provides an alternative to organic triflate based cross-coupling reactions.
- Palladium-Catalyzed Direct Cross-Coupling of Organolithium Reagents with Aryl and Vinyl Triflates,
Carlos Vila, Valentin Hornillos, Massimo Giannerini, Martin Fañanás-Mastral, and Ben L. Feringa,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201404398