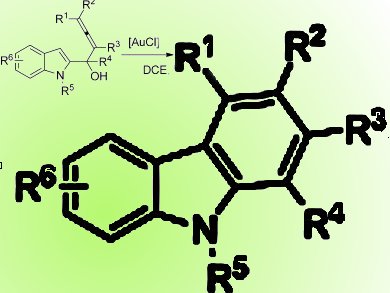
The tricyclic carbazole ring system is the core structure for a wide range of alkaloids with biological activity. Carbazole derivatives, such as N-vinylcarbazole and poly(vinylcarbazole), have applications as optoelectronic materials. Thus, carbazoles are an interesting synthetic target.
Shengming Ma, Zhejiang University, China, and colleagues report a simple and mild Au-catalyzed reaction of 1-(indol-2-yl)-2,3-allenols that provides an efficient route to various substituted carbazoles. The reaction proceeds by a selective 1,2-alkyl or aryl shift via a gold-carbene intermediate. Compared with the previous [PtCl2]-catalyzed process, the [AuCl]-catalyzed reaction represents a big step forward in terms of the scope and the selectivity of the reaction.
The team also performed DFT calculations, which supported the hypothesized 1,2-migration on the metal carbene intermediate and uncovered the co-catalytic role of the water formed in situ. The presence of water was shown to make the direct elimination of H2O a reversible stepwise proton-abstraction-donation process, thereby lowering the energy required. The calculations also suggested that the migratory preference for bulky alkyl groups is a consequence of releasing the steric strain.
- Studies on [PtCl2]- or [AuCl]-Catalyzed Cyclization of 1-(Indol-2-yl)-2,3-Allenols: The Effects of Water/Steric Hindrance and 1,2-Migration Selectivity,
Youai Qiu, Chunling Fu, Xue Zhang, Shengming Ma,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402423