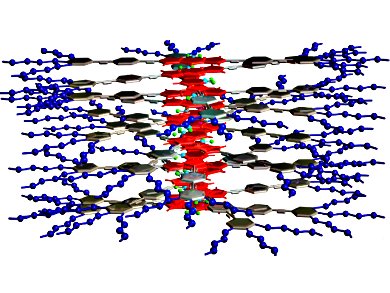
Understanding the mechanisms that govern the encapsulation of guest molecules in supramolecular assemblies could provide useful insights for the design of future drug-delivery systems.
With this in mind, Gustavo Fernández and co-workers, Universität Würzburg, Germany, investigated the self-assembly of an amphiphilic oligophenyleneethynylene (OPE)-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) derivative in the presence of different guest molecules under aqueous conditions. Interestingly, they found that the size of the guest had a dramatic effect on the aggregation mechanism and the final morphology.
Without any guest molecules, flexible fiber-like assemblies formed. When the aromatic dye tetracene was encapsulated by the assemblies, it increased the rigidity of the fibers through increased π-π and hydrophobic interactions. However, if a slightly smaller aromatic dye, anthracene, was introduced instead, the BODIPY derivatives and guest dyes co-assembled into spherical micelles through a two-step process.
-BODIPY Systems.jpg)
- H-Aggregates of Oligophenyleneethynylene (OPE)-BODIPY Systems in Water: Guest Size-Dependent Encapsulation Mechanism and Co-aggregate Morphology,
Naveen Kumar Allampally, Alexander Florian, María José Mayoral, Christina Rest, Vladimir Stepanenko, Gustavo Fernández,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402077