Silicon
While the molecular diversity associated with carbon is unmatched by its downstairs neighbor in the Periodic Table, silicon, this heavier element, does have a quite large repertoire of its own. From the silanes to the silicones and all in between. One group of silicon-containing compounds, the substituted siloles, holds great promise for the construction of conjugated polymers with intriguing photoluminescent and charge-transfer properties for photovoltaic and optoelectronic applications. Unfortunately, simple routes to polyamino-substituted 1H-siloles – which would make the perfect building blocks for these materials – have been difficult to find.
Synthesis and Reactivity of a Polyamino-substituted 1H-silole
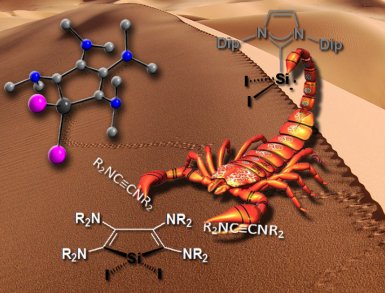
Alexander Filippou of the Institute for Inorganic Chemistry at the University Bonn, Germany, and colleagues Yury Lebedev, Ujjal Das, Oleg Chernov, and Gregor Schnakenburg, having recognized something of a recent renaissance in low-valent silicon chemistry, have found a silicon reagent, SiI2(Idip) (Idip = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene), that can undergo [2+2+1] cycloadditions with ynediamines R2N−C≡C−NR2 (R = Me, Et) to give orange-colored 2,3,4,5-tetraamino-1H-siloles. The products are water sensitive but the team’s single-crystal X-ray diffraction studies reveal them to feature a five-membered silacyclopenta-2,4-diene ring with a slight twist and an unexpected C–C bond alteration in the diene fragment.

The next step was to demonstrate what novel compounds these reagents might be used to generate. The team reacted their main focus compound with the N-heterocyclic carbene, 1,3,4,5-tetramethylimidazolin-2-ylidene, IMe4. If the iodine is then displaced from this intermediate a dicationic 1H-silole derivative is produced, which has a four-coordinate Si(IV) center. The final step to reach the thermolabile, but carbene stabilized 1-silacyclopentadien-1-ylidene is two-electron reduction with pyrophoric KC8 (potassium graphite, a material formed by melting potassium over graphite). Elemental analysis and data from proton, carbon-13 and silicon-29 nuclear magnetic resonance (NMR) spectroscopy prove the access route to these unprecedented compounds.
The team was rather surprised by the fast and selective conversion which takes place in the [2+2+1] cycloaddition given that silylenes have an ambivalent reactivity towards alkynes and form only in trace quantities siloles in their reactions with alkynes. All they can say from the preliminary mechanistic investigations is that said intermediate must be passed from one step to the highly nucleophilic ynediamine very rapidly indeed to give the product.
Future Vision
“My future vision for this chemistry is to probe the scope of this reagent as a building block for other unsaturated silacycles, to explore the chemistry of the carbene-stabilized 1-silacyclopentadien-1-ylidene and to study the chemistry and photophysical properties of the polyamino-1H-siloles,” Filippou told ChemistryViews.org.
Peter Jutzi of the University of Bielefeld, Germany, told ChemistryViews.org that the work is certainly worthy: “The carbene-stabilized SiHal2 compounds by Filippou (Hal = I) and Roesky (Hal = Cl) are very important novel substrates to enter the chemistry of low-valent and also of tetravalent silicon. Indeed, siloles are very important components of LED and other electronic materials, as shown mainly by the Japanese researchers K. Tamao and S. Yamaguchi,” he told us. “The amino-substituted siloles of Filippou might allow further progress in this field.”
- SiI2(Idip): Syntheses and Reactivity Studies of Unprecedente 2,3,4,5-Tetraamino-1H-siloles,
Yury N. Lebedev, Ujjal Das, Oleg Chernov, Gregor Schnakenburg, Alexander C. Filippou,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403108