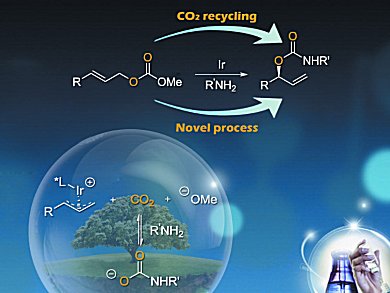
Issues of decreased carbon resources and sustainability have made the conversion of CO2 into useful chemicals a hot topic in chemistry in recent years and a great amount of attention is currently being paid to this area. At the same time, transition-metal-mediated allylation reactions have emerged as a powerful method for the regio-, diastereo-, and enantioselective formation of carbon-carbon or carbon-heteroatom bonds.
X.-M. Zhao and co-workers, Tongji University, China, have combined these two important areas of chemical study by the proposition that CO2 may be recycled by an attack of nucleophile such as an amine to induce an amidation reaction, which would then be followed by an Ir-catalyzed allylation reaction to give a branched allylic carbamate. Upon examining this proposition, the group achieved the enantioselective transformation of allyl carbonates into branched allyl carbamates by using amines and recycling CO2 in the presence of an Ir-complex and K3PO4. Products were produced in fair to excellent yields with excellent regio- (98:2) and enantioselectivity (93 % ee).
Allyl carbamates are an important class of compounds with broad interest with regards to both synthetic intermediates and biologically active molecules, therefore, a new synthetic method for their preparation is highly desirable.
- Enantioselective Transformation of Allyl Carbonates into Branched Allyl Carbamates by Using Amines and Recycling CO2 under Iridium Catalysis,
Sheng-Cai Zheng, Min Zhang, Xiao-Ming Zhao,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402388