Terpenoids constitute one of the largest natural product families, with important economic value, for example, as flavours, fragrances, spices, and drugs. Recently, a family of meroterpenoids, the psiguadials A, C, and D, were isolated from psidium guajava, an important food crop and medicinal plant found in the tropical and subtropical regions. The psiguadials are structurally related to the macrocarpals that were isolated from eucalyptus globulus, pointing towards a similar biosynthetic origin. Several members of these meroterpenoids are associated with interesting biological properties, such anti-HIV and antibacterial activity, as well as inhibition of aldol reductase and glucosyl transferase. However, their mode of action and potential molecular targets has so far remained elusive.
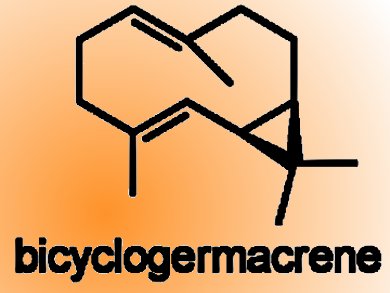
Duc N Tran and Nicolai Cramer, EPFL, Lausanne, Switzerland, describe a good-yielding, seven-step synthesis of bicyclogermacrene, a strained bicyclic and common sesquiterpene found in several essential oils. This compound was subsequently used as the key platform intermediate for a biomimetic access to several aromadendrene sesquiterpenoids, namely, ledene, viridiflorol, palestrol, and spathulenol. In addition, bicyclogermacrene is shown to be the terpene component in the synthesis of the meroterpenoids psiguadial A, C, and D.
The new-found accessibility of these compounds may enable new studies of their pharmacological activity profiles and molecular targets.
- Biomimetic Synthesis of (+)-Ledene, (+)-Viridiflorol, (–)-Palustrol, (+)-Spathulenol, and Psiguadial A, C, and D via the Platform Terpene (+)-Bicyclogermacrene,
Duc N. Tran, Nicolai Cramer,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403082
This paper will be featured in the Chemistry – A European Journal’s Young Chemists Special Issue.