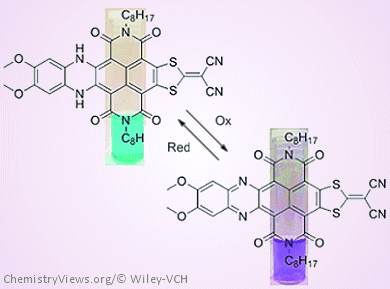
Naphthalene diimides (NDIs) are versatile aromatic compounds with enormous potential for a multitude of applications, especially in single-molecule studies, as sensors, in organic electronics, and in supramolecular architectures. The optical and electrochemical properties of NDI derivatives can be tuned by modification on the NDI core. However, there are only a few examples of NDI core substitution where groups at the C2/C3 and C6/C7 positions are different.
Hence, a team led by Steven Langford, Monash University, Australia, has looked at the synthesis, photophysical properties, and computational analysis of dissymmetrically core-extended NDIs consisting of benzo[b]phenazine and cyanoethene dithiolate substituents. These “push-pull” motifs lead to a highly-reversible redox molecular switch, that is, the fluorescence of the compounds can be switched “on and off” by changing between the oxidized and reduced forms.
The team hopes that this example of responsive materials based on dissymmetrically core-extended NDIs will form the basis of more sophisticated systems in the future.
- A Redox Switchable Dihydrobenzo[b]pyrazine Push-Pull System,
Subashani Maniam, Heather F. Higginbotham, Si-Xuan Guo, Toby D. M. Bell, Ekaterina I. Izgorodina, Steven J. Langford,
Asian J. Org. Chem. 2014, 3, 619–623.
DOI: 10.1002/ajoc.201402015