Chiral Cγ-tetrasubstituted α-amino acid derivatives, especially those bearing spirocyclic oxindole scaffolds, have significant biological and pharmacological activities. As a result, many elegant methods have been established for their enantioselective syntheses. Moreover, the chemistry of 3,3’-disubstituted oxindoles and diversely functionalized spirocyclic oxindoles has also attracted extensive research efforts because of the biological and synthetic significance of these scaffolds.
The group of Wen-Jing Xiao, Central China Normal University, Wuhan, China, reported an organocatalytic asymmetric conjugate addition of 2-oxindole-3-carboxylate esters to 2-phthalimido acrylates catalyzed by a quinine-derived thiourea.
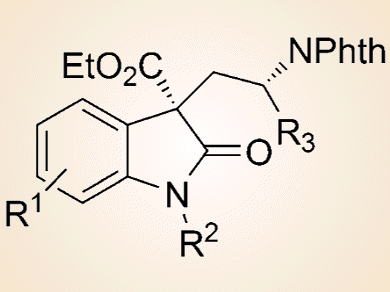
This reaction furnishes the corresponding spirocyclic oxindole-based Cγ-tetrasubstituted α-amino acid derivatives (see picture) with generally good diastereo- and enantioselectivities. Notably, both enantiomers of the products could be conveniently obtained with comparable results, which is important for biological studies of these products.
- Organocatalytic Asymmetric Conjugate Addition of 2-Oxindole-3-Carboxylate Esters to 2-Phthalimido Acrylates: Efficient Synthesis of Cγ-tetrasubstituted α-Amino Acid Derivatives,
Juan Gao, Jia-Rong Chen, Shu-Wen Duan, Tian-Ren Li, Liang-Qiu Lu, Wen-Jing Xiao,
Asian J. Org. Chem. 2014, 3, 530–535.
DOI: 10.1002/ajoc.201300292



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)