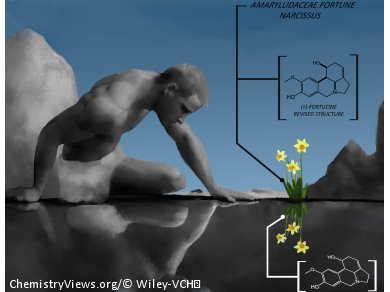
According to Greek mythology the species of plant Fortune Narcissus of Amarillydaceaes grew exactly where Narcissus drowned while he was admiring his own reflection (his mirror image) in the water.
Fortucine, isolated from the leaves of this plant, is a member of the synthetically desirable lycorine alkaloids. They have been found to possess antiviral and antitumor activities.
The story above serves as an appropriate analogy for the synthesis of the mirror-image of fortucine by Sylvain Canesi and co-workers, Université du Québec à Montréal and Concordia University, Canada. In this synthesis, the reassignment of the absolute configuration of this natural product to its mirror image was achieved by a convergent and enantioselective route initiated by the starting materials tyrosine methyl ester and 3-hydroxy-4-methoxybenzaldehyde. Two key steps mediated by a hypervalent iodine reagent. A Wipf-type strategy based on the Kita oxidative dearomatization process to provide stereoselectivity, followed by oxidative decarboxylation, were central in the synthesis.
- Asymmetric Synthesis of Fortucine and Reassignment of Its Absolute Configuration,
Marc-André Beaulieu, Xavier Ottenwaelder, Sylvain Canesi,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402323