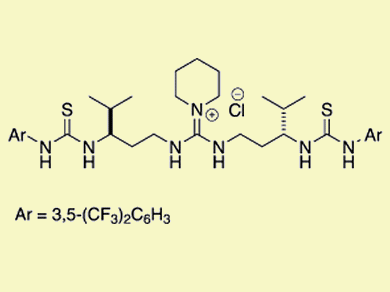
Double hydrogen-bonding interactions are key activation modes in asymmetric organocatalysis. Inspired by the relatively strong and directional hydrogen-bond donors of Jacobsen’s chiral (thio)urea catalysts and the soft and cooperative nature of enzymes, Yoshihiro Sohtome, RIKEN, Japan, and Kazuo Nagasawa, Tokyo University of Agriculture and Technology, Japan, and co-workers linked hydrogen-bond donors and molecular recognition processes in nonmetal-based enzymes to explore a series of conformationally flexible guanidine/bisthiourea organocatalysts.
The research team demonstrated that the 1,3-diamine-tethered guanidine/bisthiourea organocatalyst can be applied to diastereo- and enantioselective Michael reactions of β-dicarbonyl compounds with nitroolefins to construct two contiguous stereocenters with high diastereo- and enantioselectivity.
The group hopes that collection of the available asymmetric bond-forming reactions and in situ tuning systems with a single chiral catalyst will increase the stereodiversity and structural complexity of synthetically accessible chiral molecules.
- Asymmetric Michael Reaction of Nitroolefins with β-Dicarbonyl Compounds Catalysed by 1,3-Diamine-Tethered Guanidine-Thiourea Bifunctional Organocatalysts,
Natsuko Horitsugi, Kohei Kojima, Koji Yasui, Yoshihiro Sohtome, Kazuo Nagasawa,
Asian J. Org. Chem. 2014, 3, 445–448.
DOI: 10.1002/ajoc.201402002