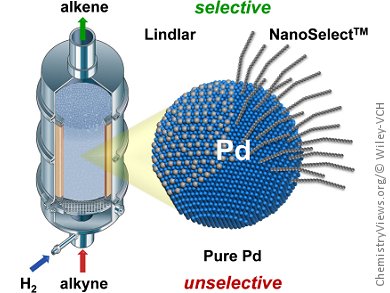
Palladium-based catalysts play a crucial role in the manufacture of a wide variety of fine chemicals, owing to their ability to partially hydrogenate alkynes to alkenes. However, to avoid over-hydrogenation to alkanes, these catalysts are often deliberately poisoned. A classic example is the Lindlar catalyst, which is partially deactivated with lead.
Alternatives that avoid the environmental challenges of working with lead have recently been developed by modifying the palladium surface with ligands instead. Javier Pérez-Ramírez, ETH Zurich, Switzerland, Núria López, Institute of Chemical Research of Catalonia, Spain, and colleagues have performed an in-depth study comparing the selectivity of these materials with bare and lead-alloyed palladium-based catalysts under continuous-flow conditions.
For some substrates, such as acetylenic compounds with isolated unsaturations and hydroxy groups, the new ligand-capped catalysts performed much better than traditional catalysts, even with ten times less palladium. On the other hand, for bulky alkynes, the lead-containing catalyst had a higher performance, owing to steric limitations from the ligands. By combining these experimental results with theoretical calculations, the researchers developed a molecular-level understanding of the selectivity patterns, to enable the development of even more advanced catalysts.
- From the Lindlar Catalyst to Supported Ligand-Modified Palladium Nanoparticles: Selectivity Patterns and Accessibility Constraints in the Continuous-Flow Three-Phase Hydrogenation of Acetylenic Compounds,
Gianvito Vilé, Neyvis Almora-Barrios, Sharon Mitchell, Núria López, Javier Pérez-Ramírez,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304795