Multifunctional Microcapsules Made from Metals and Tannic Acid
Microcapsules with a broad spectrum of applications in biomedicine, catalysis, and technology can be produced by using plant-derived, phenolic tannic acid and a variety of metals. The capsules are formed by a simple self-assembly process, and their properties can be controlled through the choice of metal, as demonstrated by a team of Australian and German researchers in the journal Angewandte Chemie.
Metals and organic molecules can combine to form coordination compounds whose structure and properties depend on the components. Examples from nature include the oxygen-binding heme groups in our red blood cells with their central iron atom or the magnesium complex at the heart of photosynthesis. Scientists have also explored the use of these types of compounds to build things, such as networked scaffold structures. A team from the University of Melbourne, the Baker IDI Heart and Diabetes Institute, Melbourne, Australia, and the University Medical Center Freiburg, Germany, is particularly interested in structures in the form of hollow capsules.
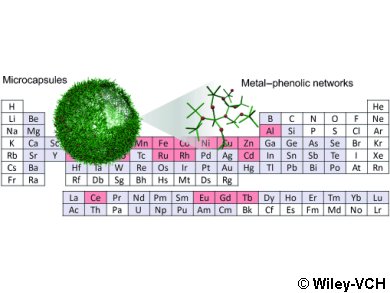
Led by Frank Caruso, these researchers haven been able to demonstrate that a single organic ligand, tannic acid, can coordinate to 18 different metals to form capsules made of metal–phenolic networks (MPNs). The metals are aluminum, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, zirconium, molybdenum, ruthenium, rhodium, cadmium, cerium, europium, gadolinium, and terbium.
The production method is simple: just mix tannic acid with a solution of the desired metal ion in the presence of a suitable substrate—in this case microparticles. Removal of the substrate leaves behind hollow microcapsules.
The properties of the capsules depend on the type and number of metal ions. For example, capsules with aluminum have a property profile suitable for drug transport: while they are relatively stable at pH values typical of blood, they come apart at the lower pH values found in some cell compartments. They could thus be used to transport a drug though the blood and release it after entering a cell.
Applications
Capsules with europium and terbium ions can be used for multicolored fluorescence labeling of biological samples, as well as for technological applications like flexible color displays. Capsules with manganese are highly promising contrast agents for magnetic resonance imaging (MRI). Capsules with radioactive copper isotopes are good tracers for positron emission tomography (PET). Properties like size, shape, and surface chemistry could be tailored to control the distribution of the capsules in the body. Capsules with radioactive copper and europium could allow for tissue samples to undergo PET followed immediately by fluorescence microscopy.
Catalysis is another possible application. The researchers were able to show that capsules with rhodium catalyze the hydrogenation of quinoline at least as well as conventional rhodium catalysts.
- Engineering Multifunctional Capsules through the Assembly of Metal–Phenolic Networks,
Junling Guo, Yuan Ping, Hirotaka Ejima, Karen Alt, Mirko Meissner,
Joseph J. Richardson, Yan Yan, Karlheinz Peter, Dominik v. Elverfeldt, Christoph E. Hagemeyer, Frank Caruso,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201311136