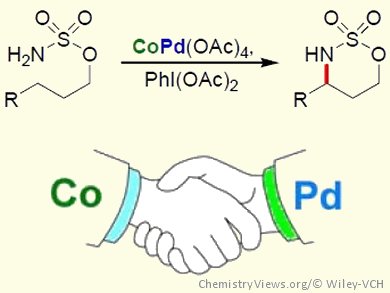
The development of new methodologies to construct C–N bonds has received much attention in recent years, owing to the large number of nitrogen-containing functional groups found in a variety of biologically active compounds. Metal-catalyzed C–N bond formation from C–H bonds is, therefore, a major area of interest.
Inspired by the chemistry of dirhodium-catalyzed C–H amination, Gary Jing Chuang, Chung Yuan Christian University, Zhongli City, Taiwan, and co-workers have developed Co/Pd-cocatalyzed C–H amination and aziridination reactions. The intramolecular C–H amination and aziridination of a range of sulfamate esters was achieved by using CoPd(OAc)4 as the catalyst and PhI(OAc)2 as the oxidant. Control experiments showed that neither Pd(OAc)2 nor Co(OAc)2 could catalyze the reaction alone, demonstrating that the presence of both metals is essential for reactivity. The chemoselectivity of the CoPd(OAc)4-catalyzed amination was also investigated and showed a preference for tertiary and benzylic C–H bonds over secondary C–H bonds in the formation of six-membered cyclic products.
This research could potentially be applied to the synthesis of 1,3-amino alcohols, β-amino acids, and other 1,3-difunctionalized derivatives.
- Cooperative Effect of Two Metals: CoPd(OAc)4-Catalyzed C–H Amination and Aziridination,
Guan-Hao Huang, Jian-Min Li, Jing-Jyun Huang, Jyun-Dai Lin, Gary Jing Chuang,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304633