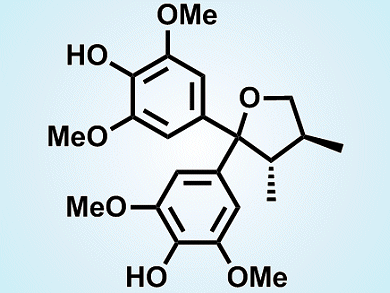
(–)-Sacidumlignan D is a structurally unique lignan that is isolated from the plant Sarcostemma acidum (Roxb.). The local residents of Hainan Island of China apply the plant as folk medicine to treat chronic coughs and postnatal hypogalactia.
A team led by Ran Hong, Shanghai Institute of Organic Chemistry, and Xueshun Jia, Shanghai University, both China, have developed a concise route to synthesize the antipode of the natural product, (+)-sacidumlignan (pictured). A zinc-mediated crotylation of a protected diarylketone and subsequent lactonization were used to establish the carbon framework. Then, with the guidance of the proposed biogenesis, the team implemented the key step of a kinetic resolution catalyzed by a chiral phosphoric acid ((S)-TRIP) to recover the target diaryl (+)-diol in high enanotiomeric excess. The (+)-diol was then further manipulated to finalize the synthesis of (+)-sacidumlignan D (see scheme below; TBAF=tetra-n-butylammonium fluoride, TBS = tert-butyldimethylsilyl, TFA = trifluoroacetic acid).
The origin of stereoselectivity during etherification might be attributed to a chiral ion-pair intermediate.

The group hopes this asymmetric etherification method could find new applications in asymmetric synthesis of other natural products.
- Kinetic Resolution of Diols via Etherification Catalyzed by a Chiral Phosphoric Acid: Concise Synthesis of (+)-Sacidumlignan D,
Changmin Xie, Donghu Bai, Sha-Hua Huang, Xueshun Jia, Ran Hong,
Asian J. Org. Chem. 2014, 3, 277–280.
DOI: 10.1002/ajoc.201300250