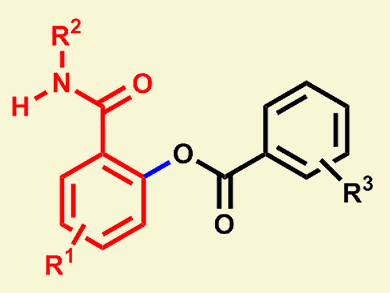
A highly atom-economical and environmentally friendly manner to effectively form various chemical bonds, such as C–C, C–N, C–X, and C–O, is the metal-catalyzed chelation-assisted functionalization of ortho-C–H bonds of aromatics with nucleophiles by C–H bond activation. Palladium, rhodium, and ruthenium complexes are widely used as catalysts for these types of reactions. In the presence of these catalysts, C–C, C–X, and C–N bond formation have been extensively studied; C–O bond formation, however, has not been well explored.
Masilamani Jeganmohan and Kishor Padala, Indian Institution of Science Education and Research, Pune, India, have demonstrated C–O bond formation by the highly regioselective ortho-benzoxylation of benzamides with substituted benzoic acids in the presence of a ruthenium catalyst, [{RuCl2(p-cymene)}2], AgSbF6, and (NH4)2S2O8 in 1,2-dichloroethane. The catalytic reaction worked very efficiently with various functional-group-substituted benzamides and benzoic acids yielding the ortho-benzoxylated benzamides in good to excellent yields. Furthermore, the formed benzoxylated benzamides were subjected to either a Ru-catalyzed Heck-type alkenylation with alkenes in water or treated with acid or base to produce ortho-hydroxy N-alkyl benzamides.
- ortho-Benzoxylation of N-Alkyl Benzamides with Aromatic Acids Catalyzed by Ruthenium(II) Complex,
Kishor Padala, Masilamani Jeganmohan,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304646