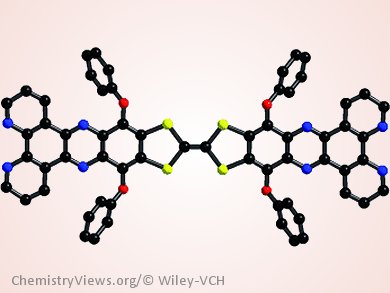
Tetrathiafulvalene (TTF), as a π-electron donor, has attracted particular attention within the context of materials chemistry towards molecular (opto)electronics. However, large π-conjugated TTF derivatives with structurally rigid and planar configurations have rarely been studied owing to limited preparative accessibility.
In response to this problem, Shi-Xia Liu and co-workers, Universität Bern, Switzerland, have introduced a concept for the annulation of TTFs to diverse π-electron acceptors by a Schiff base reaction. For instance, direct annulation of two dipyrido[3,2-a:2’,3’-c]phenazine units to the TTF core yields a large π-conjugate as a bridging ligand. Its coordination ability is exemplified by the formation of a stable dinuclear ruthenium (II) complex.

These electrochemically amphoteric compounds exhibit various intense intramolecular charge-transfer absorption bands in different oxidation states. Specifically, an unprecedented TTF•+ radical cation dimerization and oligomerization can be identified upon oxidation of the bridging ligand owing to strong intermolecular π–π interactions.
- Large π-Conjugated Chromophores Derived from Tetrathiafulvalene,
Hongpeng Jia, Jie Ding, Andreas Hauser, Silvio Decurtins, Shi-Xia Liu,
Asian J. Org. Chem. 2014, 3, 198–202.
DOI: 10.1002/ajoc.201300144