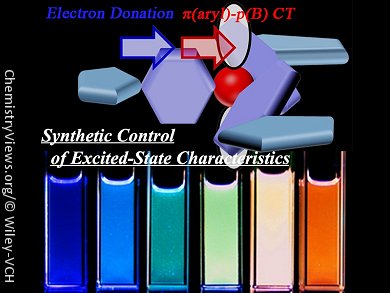
Highly emissive multicolor chromophores are important for the development of materials that can be applied to organic light-emitting diodes (OLEDs), and imaging and sensing devices. A variety of triarylborane derivatives, such as polymers and transition-metal complexes, with relatively large π-electron systems have been successfully utilized for the development of such materials.
Akitaka Ito and Noboru Kitamura, Hokkaido University, Japan, and co-workers have developed a new series of triarylborane derivatives, in which the spectroscopic and photophysical properties can be controlled by the nature of the triarylborane core and peripheral electron-donating groups. Charge-transfer interactions between the triarylborane core and the peripheral electron-donating groups of these derivatives result in tunable fluorescence color (from blue to red). The introduction of strong electron-donating groups makes the fluorescent excited state lower in energy, and the choice of triarylborane core determines the effect of solvent polarity on the fluorescence quantum yield and lifetime. Further electrochemical, spectroscopic, and photophysical investigations of the triarylborane derivatives demonstrated that the HOMO and LUMO of each derivative were determined by the nature of the electron-donating groups and the triarylborane core, respectively.
The researchers hope that this study will contribute towards future triarylborane-based materials and imaging devices.
- Synthetic Control of Spectroscopic and Photophysical Properties of Triarylborane Derivatives Having Peripheral Electron-Donating Groups,
Akitaka Ito, Kazuyoshi Kawanishi, Eri Sakuda, Noboru Kitamura,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304207