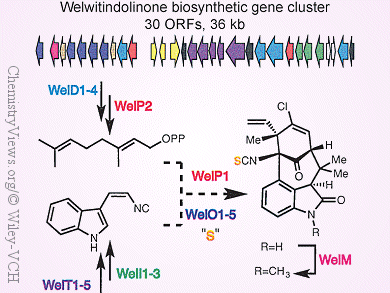
Welwitindolinones and related hapalindoles, fischerindoles, and ambiguines are a large family of terpenoid indole alkaloids isolated from the Stigonematalean cyanobacteria Fischerella sp. and Hapalosiphon sp. Owing to their intriguing structural complexities and diverse biological activities, the synthesis of hapalindole-type natural products have been studied extensively. However, their biochemical origins, at both the genetic and molecular levels, are virtually unknown.
Xinyu Liu and colleagues, University of Pittsburgh, PA, USA, have identified and characterized the biosynthetic gene cluster of a welwitindolinone (wel) from the cyanobacterium Hapalosiphon welwitschii.
Further comparative analysis with an ambiguine (amb) biosynthetic gene cluster recently published by the same group [1], revealed a striking similarity between the wel and amb pathways in the assembly of early biosynthetic intermediates. This similarity implies that the structural diversity within hapalindole-type molecules has most likely been derived from the late-stage enzymatic actions of non-heme iron-dependent oxygenases, in particular Rieske-type oxygenases.
These findings, thus, provide the genetic and molecular basis for future studies of novel enzymes associated with the biosynthesis of hapalindole-type natural products and the rational engineering of these pathways for the production of therapeutically important alkaloids in vivo.
- Identification and Characterization of a Welwitindolinone Alkaloid Biosynthetic Gene Cluster in Stigonematalean Cyanobacterium Hapalosiphon welwitschii,
M. L. Hillwig, H. A. Fuhrman, K. Ittiamornkul, T. J. Sevco, D. H. Kwak, X. Liu,
ChemBioChem 2014, 15, 665–669.
DOI: 10.1002/cbic.201300794
[1] M. L. Hillwig, Q. Zhu, X. Liu, ACS Chem. Biol. 2014, 9, 372–377. DOI: 10.1021/cb400681n