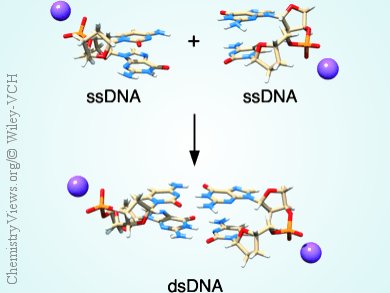
Giampaolo Barone, Università di Palermo, Italy, F. Matthias Bickelhaupt, VU University, Amsterdam, The Netherlands, and colleagues computationally investigated the structure and stability of B-DNA as a function of nucleic acid composition. It is believed that the B form is the naturally occurring form of the DNA into the living cells.
This is the first quantum chemical study that copes with these large model systems using dispersion-corrected DFT and implicit solvation (COSMO). The team obtained optimized geometries with B-DNA conformation through the inclusion of implicit water solvents and of sodium counterions in the DNA models, to neutralize the negative charge of the phosphate groups.
The results allowes a comparison of the relative stability of isomeric single and double strands. Calculation of the energy of Watson–Crick pairing of complementary single strands to form double-helical structures, allowed the compilation of a stability trend of the double-helix formation energy. This trend shows that the formation energy increases with the number of hydrogen bonds per base pair, , namely, two for adenine and thymine,and three for guanine and cytosine. However, more subtle effects depending on the order of bases within a strand from the 5’- to the 3’-terminus are superimposed on this main trend.
 B-DNA Structure and Stability as Function of Nucleic Acid Composition: Dispersion-Corrected DFT Study of Dinucleoside Monophosphate Single and Double Strands,
B-DNA Structure and Stability as Function of Nucleic Acid Composition: Dispersion-Corrected DFT Study of Dinucleoside Monophosphate Single and Double Strands,
Giampaolo Barone, Célia Fonseca Guerra, F. Matthias Bickelhaupt,
ChemistryOpen 2013, 2, 186–193.
DOI: 10.1002/open.201300019