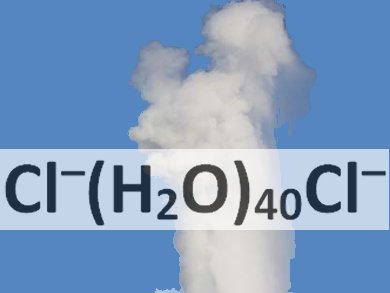
Application of Dichloride Pairs
A collaboration between the teams of Alexander Boldyrev and Alexander Ivanov of Utah State University, Logan, USA, and Gernot Frenking of Philipps-Universität Marburg, Germany, has looked at the microsolvation of chloride pairs. The researchers have found that in silico at least it takes approximately forty water molecules to surround a pair of negative chloride ions with a sufficiently robust cluster that dissociation is precluded. In other words, in the chemical species Cl–(H2O)nCl– n must be greater than 40 so that the two negative ions do not repel each other Coulombically in the gas phase.
The researchers suggest that the association of such anion pairs in the gaseous state could play an important role in nucleation of industrial steam and so have implications for chemical engineering. The phenomenon may also have implications for our understanding of geothermal steam from hot springs and geysers and their impact on local climate and ecosystems. The work might also take us a step closer to an understanding of the behavior of solutions, given that these large clusters can be considered as proxies for the solution phase.
Structure of Dichloride Anion Pair
Chloride ions have to be among the most familiar of chemical species, we might assume, being present in common salt alongside sodium ions. And yet, the dichloride anion pair is not so well known nor well studied. Indeed, according to Boldyrev and colleagues, there have been no reports of investigations of this species in the gas phase despite the presumption that it will exist and play an important role in the behavior of chloride-containing systems.
The team has now used systematic ab initio study of microsolvation of the dichloride anion pair to reveal a stepwise solvation mechanism as the number of water molecules associated with the pair is increased from two to ten. Intriguingly, they found that the “lowest” structure for dichloride hexahydrate closely resembles the cubic water octamer with two water molecules swapped out for two chloride anions at opposite corners of the cube.
It is the stabilization of the chloride pair by at least thirty-six water molecules that is of particular interest. With this number of water molecules in the cluster, cyclic structures form that bridge the chloride ions. Moreover, with thirty-six or forty water molecules one can envisage the system as being the tiniest possible droplet of aqueous dichloride solution existing in a state analogous to the bulk, the team suggests.
Bizarre, adding more water molecules to this level does not disrupt the octahedral pattern the team hypothesizes at lower numbers. As such, such relatively “simple” clusters might lighten the computational load when one wishes to investigate the behavior of dichloride ions in bulk solution.
The team points out that, strictly speaking, the only stable anion pairs of chloride are, in fact, hydrogen-bonded clusters of chloride ions and “n” water molecules. So, they are not genuine dianions, but anion clusters that are large enough that the hydrogen bonds of the outer water molecules are stronger than the Coulombic repulsion between two chloride ions. Nevertheless, this is an important finding.
Nucleation of Industrial and Geothermal Steam
“The understanding of many physical and chemical processes that involve fluids in the Earth’s crust and reactions in oceanic and atmospheric environments requires a detailed knowledge of the mechanisms in which ions and molecules are solvated”, Boldyrev says. Hydrated chloride ions being the most common as presumed to play important roles in a wide variety of chemical, environmental, and biological processes. “We think that the association of stable gaseous Cl−−Cl− anion pairs may play an important role in nucleation of industrial steam or geothermal steam from the Geysers wells”, Boldyrev explains.
Molecular Models for Understanding Processes in Bulk Solution
Because of the enormous complexity of solutions, in particular, aqueous solutions, it is very challenging to obtain a molecular picture of the structure of solution. Hence, physical chemists, such as Lai-Sheng Wang of Brown University, Providence, RI, USA, have used water clusters and hydrated water clusters around an anion or cation as molecular models to obtain insight about the bulk solution.
“The current work by Boldyrev and colleagues presents a systematic study of the solvation of two anions. This is definitely a very interesting result, because we all know that like charges repel each other”, Wang told ChemistryViews. “Thus, these types of dianion clusters help us understand the different types of interactions within the clusters, which will be useful for bulk solutions, that is, like charge repulsion, dielectric shielding, solvent–solute interactions, and solvent–solvent interactions. In particular, I find the result of the thirty-six water dichloride cluster is inspiring, which may stimulate experimentalists to search for such species in the gas phase.”
- Stabilization of a Cl––Cl– Anion Pair in the Gas Phase: Ab Initio Microsolvation Study,
Alexander S. Ivanov, Gernot Frenking, Alexander I. Boldyrev,
J. Phys. Chem. A 2014.
DOI: 10.1021/jp4123997