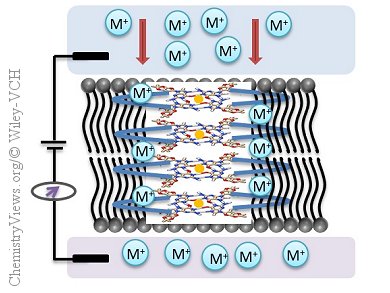
Transmembrane proteins facilitate the transport of ions across lipid bilayers in biological membranes. In recent years, the synthesis of compounds that can mimic the structural aspects of these transmembrane proteins has received much attention. For example, biomimetic nanochannels have been developed for use as drug delivery systems, antimicrobial agents, and biosensors. However, the preparation of these ion channels generally involves multistep linear syntheses, resulting in low overall yields.
Claudia Steinem, Georg August University Göttingen, Germany, and Jyotirmayee Dash, Indian Association for the Cultivation of Science, Kolkata, India, and co-workers have developed self-assembled “click” ion channels. For this they used a high yielding azide–alkyne cycloaddition between lipophilic guanosine azides and guanosine alkynes with a variety of covalent linkers. The ion-channel activity of these diguanosine derivatives was investigated by using voltage-clamp experiments, which showed that discrete channels, with stable and large pores, were formed. These diguanosine derivatives modulated the traffic of ions across a phospholipid bilayer, exhibiting a variation in conductance spanning three orders of magnitude (pS to nS). The pore size and conductance properties of these diguanosine derivatives could be controlled by modifying the “click” linker.
This new modular approach could lead to synthetic ion channels with increased diversity, pore size, and ionic selectivity.
- Triazole-Tailored Guanosine Dinucleosides as Biomimetic Ion Channels to Modulate Transmembrane Potential,
Y. Pavan Kumar, Rabindra Nath Das, Sonu Kumar, Ole Mathis Schütte, Claudia Steinem, Jyotirmayee Dash,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304530