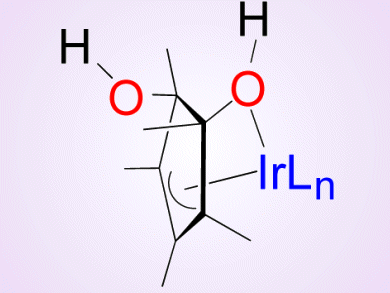
The oxidation of water to molecular oxygen is an important reaction in the frame of artificial photosynthesis, solar fuels, and a sustainable energy future. Iridium complexes have recently gained attention as effective precatalysts for this transformation.
To gain deeper understanding of this process, Alceo Macchioni, University of Perugia, Italy, and colleagues aimed at intercepting the intermediates of the oxidative transformation of [Cp*Ir(bzpy)NO3] (Cp* = pentamethylcyclopentadienyl, bzpy = 2-benzoylpyridine), which is a competent catalyst for water oxidation.
They succeeded in intercepting and characterizing three intermediates that are formed when oxidants of different nature (H2O2, CAN, and NaIO4) are used. Consequently, the oxidative transformation appears to be rather general.

The researchers observed the progressive oxidation of Cp*, whereas the benzoylpyridine ancillary ligand remained intact. The isolated intermediates and their mixtures were found to remain active in water-oxidation catalysis.
These findings will be useful for the design of future novel catalysts.
- Transformation of a Cp*–Iridium(III) Precatalyst for Water Oxidation when Exposed to Oxidative Stress,
C. Zuccaccia, G.Bellachioma, O. Bortolini, A. Bucci, A. Savini, A. Macchioni,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304412