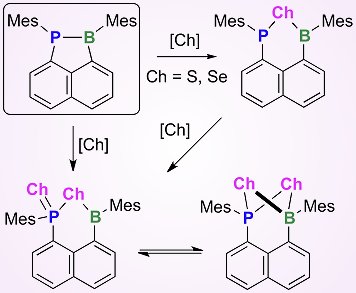
The chemistry of phosphine–boranes (R3P–BR3) bearing a P–B coordinate bond has attracted a lot of attention in recent years, especially from the viewpoint of frustrated Lewis pairs. Of particular interest are phosphine–boranes that contain a rigid linker, such as a 1,8-naphthalene core. This rigid linker is useful for the investigation of the steric nature of P–B coordinate bonds.
Takahire Sasamori, Norihiro Tokitoh, Kyoto University, Japan, and colleagues have reported the synthesis of 1-phospha-2-boraacenaphthene derivatives by the reduction of diarylboryl-8-dichlorophosphinonaphthalene compounds with elemental magnesium. Chalcogenation reactions of these stable phosphine–boranes with elemental sulfur or selenium afforded unique heterocycles, possessing a P–Ch–B (Ch = chalcogen) moiety tethered to the naphthalene skeleton. These compounds are the first examples of heterocycles with phosphorus, boron, and chalcogen atoms in a phenalene backbone. Spectroscopic and X-ray analyses of these derivatives revealed some interesting behavior. In particular, some examples display a unique dynamic behavior in solution, such as two selenium atoms undergoing facile exchange with each other via an intermediate bearing a P(μ-Se2)B four-membered ring system.
The researchers hope that this study will be useful for the synthesis of unique heterocyclic systems by utilizing a P–B bond.
- Synthesis and Structure of a 1-Phospha-2-boraacenaphthene Derivative and Its Chalcogenation Reactions,
Akihiro Tsurusaki, Takahiro Sasamori, Norihiro Tokitoh,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304644