Lithium-ion batteries (LIBs) are among the most promising energy-storage devices for applications ranging from portable electronics to electric vehicles owing to their high efficiency and energy density. Transition-metal oxides hold great promise toward high-energy density negative electrodes for LIBs owing to their significantly higher theoretical capacities compared with graphite-based electrodes (372 mA h g–1) currently used in commercial LIBs. However, most metal oxides have low electrical and ionic conductivities, leading to low electrochemical performance. In addition, some transition-metal oxides undergo large volume changes upon charge/discharge, introducing volumetric stress in the electrode and leading to poor cycling stability.
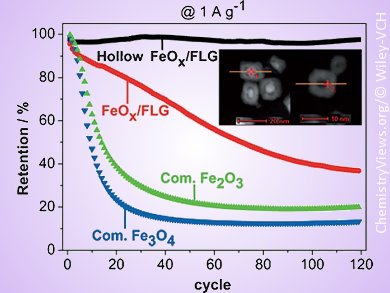
Zhenyu Sun, Edgar Ventosa, Ruhr-University Bochum, Germany, and colleagues have developed a template-free synthesis for hollow and yolk-shell iron oxide (FeOx) nanoparticles (NPs) sandwiched between few-layer graphene (FLG) sheets. The material’s properties were evaluated as negative electrode material for LIBs. Electrochemical studies showed that the carbon content of electrodes is of crucial importance for the cyclability because carbon reduces the volumetric stress and enhances the electrical conductivity. As such, use of pristine FLG improved the capacity retention. For a low carbon loading of 18 wt %, the presence of metallic iron in the hollow FeOx/FLG decreased the initial reversible capacity but it significantly improved the capacity retention up to 97 % even after 120 cycles at 1000 mA g–1 in the voltage range of 0.1–3.0 V. Further oxidation of the inactive metallic iron to active iron oxide by annealing in air led to higher initial capacity: the oxidized hollow FeOx/FLG stored reversibly about 900 mA h g–1 over 40 cycles with a capacity retention close to 100 %, but a decay in capacity occurred upon further cycling.
- Hollow and Yolk-Shell Iron Oxide Nanostructures on Few-Layer Graphene in Li-Ion Batteries,
Zhenyu Sun, Kunpeng Xie, Zi An Li, Ilja Sinev, Petra Ebbinghaus, Andreas Erbe, Michael Farle, Wolfgang Schuhmann, Martin Muhler, Edgar Ventosa,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201303723