Targeting the Non-Mevalonate Terpene Biosynthetic Pathway
Two of the most urgent challenges for scientists are the battles against food shortages and infectious diseases like malaria. Unfortunately, both the herbicides used to protect plants and the anti-infectives that shield us from disease rapidly lose their effectiveness as the target organisms develop resistance. In order to benefit both fields at once, scientists tested lead compounds from agrochemical research against infectious germs as well. In this way, a team of German and Swiss researchers has found a new candidate that may work against malaria, as they report in the journal Angewandte Chemie.
“Recently, enzymes from the non-mevalonate terpene biosynthetic pathway have been identified as attractive target structures with novel modes of activity for the development of herbicides and drugs against infectious diseases,” explains François Diederich from the ETH Zurich, Switzerland. “This biosynthetic pathway is found in many human pathogens and in plants, but does not occur in mammals.” Correspondingly, an inhibitor should only have a toxic effect on pathogens and plants, not humans. Diederich and his co-workers at the ETH, TU Munich, BASF-SE, the University of Hamburg, the Swiss Tropical Institute STPHI in Basel, and TU Dresden have now discovered new inhibitors and characterized the ways in which they work.
Pseudilins Inhibit Enzyme IspD
By using high-throughput screening methods, the researchers of BASF SE led by Matthias Witschel tested about 100,000 compounds for an inhibitory effect against plant IspD, an enzyme of the aforementioned non-mevalonate terpene biosynthetic pathway – and found several hits. The most interesting compounds are pseudilins, highly halogenated alkaloids from marine bacteria, and have a significant inhibitory effect on IspD, as researchers at the TU Munich led by Michael Groll demonstrated in NMR-based tests and researchers at the University of Hamburg led by Markus Fischer showed in photometric tests. Says Groll: “Interestingly, the chemical scaffold of the pseudilins is completely different from that of a previously discovered IdpD inhibitor. This suggests that the mode of action should also be different.”
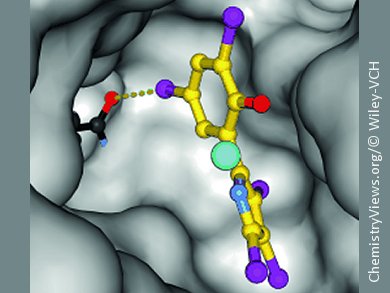
To research this mechanism, Andrea Kunfermann from Groll’s team synthesized cocrystals of the pseudilins and IspD enzymes and examined them by X-ray crystallography. This showed that the pseudilins bind to an allosteric pocket in the enzyme. Halogen atoms in the pseudilins build up halogen bridges to the enzyme, which are, in addition to metal-ion coordination, responsible for the strong binding. Occupation of this pocket changes the shape of the enzyme so that a cosubstrate required for proper functioning of the enzyme can no longer dock at the binding site in the active center.
“The pseudilins demonstrated herbicidal activity in plant assays and were active against Plasmodium falciparum, the pathogen that causes Malaria tropica and is dependent on the non-mevalonate biosynthesis pathway for survival,” reports Diederich. The researchers hope to use this as a new starting point for malaria treatment.
- Pseudilins: Halogenated, Allosteric Inhibitors of the Non-Mevalonate Pathway Enzyme IspD,
Andrea Kunfermann, Matthias Witschel, Boris Illarionov, René Martin, Matthias Rottmann, H. Wolfgang Höffken, Michael Seet, Wolfgang Eisenreich, Hans-Joachim Knölker, Markus Fischer, Adelbert Bacher, Michael Groll, François Diederich,
Angew. Chem. Int. Ed. 2014,
DOI: 10.1002/anie.201309557