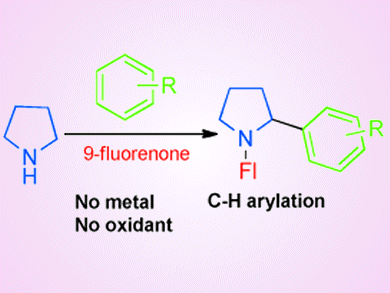
Arylated pyrrolidine-containing scaffolds have tremendous value in medicine and chemistry. Therefore, various methods for their synthesis exist, primarily through functionalization of pyrrolidine with a stoichiometric/catalytic amount of metal-based reagent or oxidant. Until now, the synthetic challenge to access these compounds through direct arylation of pyrrolidine without using organometallic reagents and/or oxidants that produce unwanted byproducts remained.
Chandan K. Jana and co-workers, Indian Institute of Technology Guwahati, Assam, India, have discovered a metal- and oxidant-free method for direct sp3 C–H arylation of pyrrolidine by a three component reaction. Upon simple heating, a solution of a nucleophilic aromatic compound, 9-fluorenone, and pyrrolidine gives arylated pyrrolidines and water as the only byproduct. The method is regioselective for the arylated product even on a multigram scale.
Moreover, this is an approach for C–H arylation based on reactivity that differs from the reactivity of iminium ions in Mannich or Betti reactions. Further investigations on the scope and mechanism of the reaction are ongoing.
 Metal- and Oxidant-Free Direct sp3 C–H Arylation of Pyrrolidine,
Metal- and Oxidant-Free Direct sp3 C–H Arylation of Pyrrolidine,
Surajit Haldar, Sujit Mahato, Chandan K. Jana,
Asian J. Org. Chem. 2014, 3, 44–47.
DOI: 10.1002/ajoc.201300233