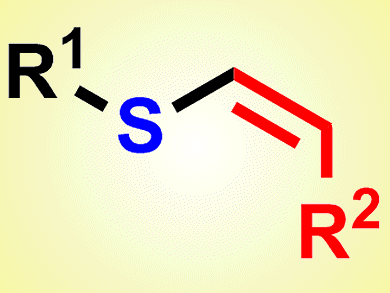
Vinyl sulfides are a class of important synthetic intermediates in organic synthesis. There are many reports on the selective synthesis of vinyl sulfides, but these methods suffer from limitations, not least the difficulties encountered in handling the foul-smelling thiols, as well as limited reaction conditions and substrate scopes. Thus, a general and simple method for the synthesis of vinyl sulfides, especially Z-vinyl sulfides, remains a challenge to be addressed.
Chun Cai and his group, Nanjing University of Science and Technology, China, have suggested a solution to this problem. They reported the preparation of vinyl sulfides by a three-component reaction of organohalides, thiourea, and alkynes. Two carbon–sulfur bonds are formed in one pot in this palladium-catalyzed system. The new protocol is mild and, importantly, free of foul-smell thiols. The products obtained are the cis-configured anti-Markovnikov products.

- Palladium-Catalyzed Odorless One-Pot Synthesis of Vinyl Sulfides from Organohalides, Thiourea, and Alkynes,
Jie Feng, Guoping Lu, Meifang Lv, Chun Cai,
Asian J. Org. Chem. 2014.
DOI: 10.1002/ajoc.201300187