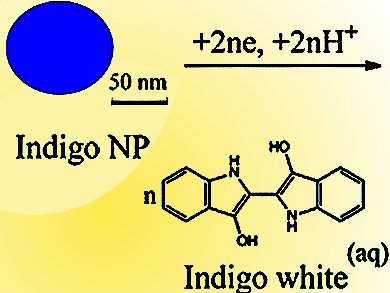
Reduction of solid indigo nanoparticles (NPs) to form soluble leuco-indigo is an important process associated with the use of indigo as a textile dye. In addition, indigo NPs, as well as other organic NPs, are important in (bio)-medical applications and food technology. However, the reactivity of such materials at the nanoscale is poorly understood. A fundamental understanding of the electrochemical interfaces associated with organic NPs is of significant interest for emerging applications.
Richard Compton and co-workers, Oxford University, UK, have studied the mechanism, rate-determining step, and charge-transfer kinetics of organic NPs by using the reductive dissolution of indigo NPs as a model system. The electron-transfer kinetics of the individual particles were identified by studying the collisions of indigo NPs with a carbon microelectrode. Analysis indicated that the indigo NPs exhibit irreversible kinetics, with charge-transfer being the rate-determining step. The authors have also proposed a mechanism, in which protonation and detachment of the reduced molecules from the NPs occur after the rate-limiting charge-transfer process.
This new insight into the mechanism associated with electron transfer to organic NPs is expected to have a significant impact on organic NP research, and to benefit the development of a wide range of applications.
 Organic Nanoparticles: Mechanism of Electron Transfer to Indigo Nanoparticles,
Organic Nanoparticles: Mechanism of Electron Transfer to Indigo Nanoparticles,
Wei Cheng, Christopher Batchelor-McAuley, Richard Compton,
ChemElectroChem 2014.
DOI: 10.1002/celc.201300233
ChemPubSoc Europe’s new journal ChemElectroChem is dedicated to covering the entire scope of pure and applied electrochemistry.