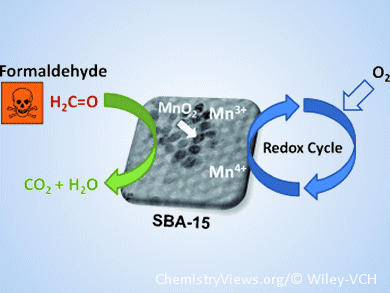
Toxic formaldehyde gas, which can be found in trace amounts (<1 ppm) in indoor air, can cause serious health problems. In an advance in formaldehyde conversion chemistry, Jean-François Lamonier and co-workers, Lille University of Science and Technology, France, have created mesoporous silica-confined MnO2 nanoparticles as a highly active catalyst, with complete selectivity towards carbon dioxide.
The heterogeneous catalyst is an excellent noble-metal-free alternative to titania-supported platinum, the hitherto most active material for formaldehyde oxidation. Furthermore, the Mn-based process requires limited energy consumption, with operating temperatures as low as 130 °C. Confined-space crystallization of MnO2 on a SBA-15 silica support produces the active material as a hexagonal array of size-adjustable mesopores, possibly with a second microporous network. Mn valence state is integral to the catalytic activity: materials with high Mn4+ content show high activity, whereas a decrease in the mean Mn oxidation state is followed by a decrease in catalytic activity.
Mn-containing nanocomposites could substitute noble metal catalysts not only in the oxidation of formaldehyde but also of other volatile organic compounds.
- Mesoporous Silica-Confined Manganese Oxide Nanoparticles as Highly Efficient Catalysts for the Low-Temperature Elimination of Formaldehyde,
Rémy Averlant, Sébastien Royer, Jean-Marc Giraudon, Jean-Pierre Bellat, Igor Bezverkhyy, Guy Weber, Jean-François Lamonier,
ChemCatChem 2013.
DOI: 10.1002/cctc.201300544