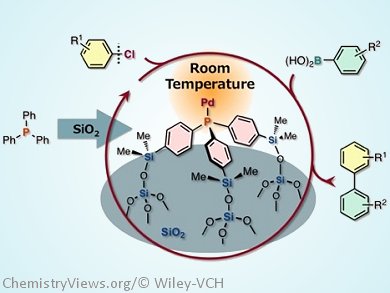
A new type of silica-supported phosphane for use in Pd-catalyzed cross-coupling reactions has been developed. This material, which features a Ph3P-type core tripodally immobilized on a silica surface (see picture), has been prepared by Masaya Sawamura and his group, Hokkaido University, Sapporo, Japan, by means of a straightforward synthetic route, the effort and cost of which is comparable to that of most sophisticated homogeneous phosphane ligands.
From spectroscopic analysis and investigation of ligand effects in the catalytic applications, they found that tripodal immobilization constrains the mobility of the phosphane molecule and causes the lone pair on the phosphorus atom to face in the direction perpendicular to the support, resulting in the selective formation of a 1:1 metal–phosphane species that is free from unfavorable steric repulsions caused by the silica surface.
When tested for catalytic activity, this Pd and the silica-supported tripod phosphane material was shown to catalyse Suzuki–Miyaura coupling reactions with unactivated chloroarenes at room-temperature.
- Tripod Immobilization of Triphenylphosphane on a Silica-Gel Surface to Enable Selective Mono-Ligation to Palladium: Application to Suzuki–Miyaura Cross-Coupling Reactions with Chloroarenes,
T. Iwai, R. Tanaka, T. Harada, M. Sawamura,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304081