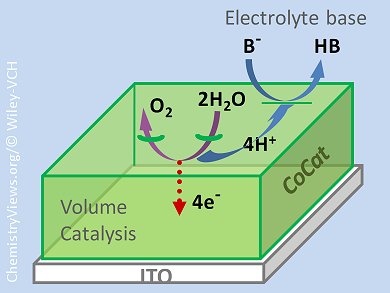
The water-oxidation reaction, one of the half-reactions in water splitting, promises to be a key technology in the long-awaited hydrogen economy. Amorphous-oxide catalysts based on cobalt, an abundant first-row transition metal, show great activity in the water-oxidation reaction under benign conditions and can be easily obtained by electrodeposition.
Holger Dau, Freie Universität Berlin, Germany, and co-workers found surprising results about how these catalysts operate. The accepted view of cobalt-based oxide catalysts is one of a solid metal-oxide film with catalysis occurring at the interface between the solid material and the bulk electrolyte. However, Dau and his team measured the catalytic rate for films of different thicknesses and found that, at low overpotentials, the rate was directly proportional to the catalyst volume. This proves that, rather than catalysis occurring only at a crystal surface, there are reactive sites throughout the bulk of the film. Catalytic activity increases proportionally to film thickness although there is a limit beyond which activity does not increase further.
This shows that, for water-oxidation catalysts, more is actually more. These results should be important in the practical application of these catalysts.
- Water Oxidation by Amorphous Cobalt-Based Oxides: Volume Activity and Proton Transfer to Electrolyte Bases,
Katharina Klingan, Franziska Ringleb, Ivelina Zaharieva, Jonathan Heidkamp, Petko Chernev, Diego Gonzalez-Flores, Marcel Risch, Anna Fischer, Holger Dau,
ChemSusChem 2013.
DOI: 10.1002/cssc.201301019