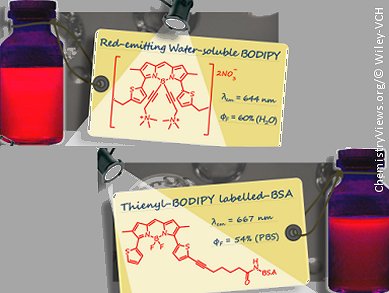
There is an increasing demand for red-emitting fluorescent dyes mainly for applications such as antibody and protein labeling. Raymond Ziessel and co-workers at the Université de Strasbourg, France, have succeeded in synthesizing three new red-emitting and water-soluble thienyl–borondipyrromethene (BODIPY) dyes.
BODIPY compounds are normally hydrophobic, so to improve the water solubilty of the dyes, which is important for biological applications, trimethyl(propargyl)ammonium groups have been introduced into the compounds. This was achieved through cross-coupling reactions or Grignard reactions with dimethylaminopropyne.
The resulting thienyl–BODIPY dyes display promising emissive properties in the red part of the visible spectrum. The usefulness of such dye frameworks was demonstrated by the controlled grafting of them onto bovine serum albumin (BSA). High quantum yields in phosphate buffered saline at pH 7.4 were observed, whereas aggregation at low loading ratios was not.
- Synthesis of Water-Soluble Red-Emitting Thienyl-BODIPYs and Bovine Serum Albumin Labeling,
A. Poirel, P. Retailleau, A. De Nicola, R. Ziessel,
Chem. Eur. J. 2014,
DOI: 10.1002/chem.201303988