Dicarboxylic acids are an important class of biologically relevant anions. They can act as biomarkers, signifying the degree of enzymatic activity in metabolism. In fact, the excretion of clinically abnormal levels of dicarboxylic acids in human urine indicates a metabolic disorder.
For rapid and general screening of metabolic disorders it is desirable to measure the total concentration of dicarboxylic acids. To this end, Seiji Shinkai, Kyushu University, Japan, and colleagues have developed a fluorescent chemosensor.
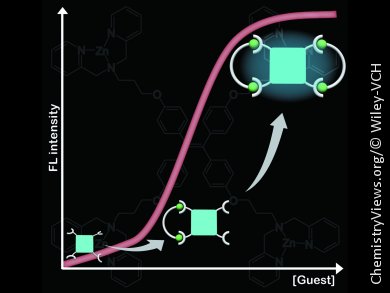
The team uses tetraphenylethene decorated with zinc–dipicolylamine groups (TPE-Zn), which is weakly fluorescent in aqueous solution. Fluorescence (FL) was switched on upon addition of L-tartarate and a strong blue emission was observed. The FL wavelength gradually shifted to shorter wavelengths, from 463 to 445 nm, with increasing L-tartarate concentration. Dynamic light scattering measurements suggested that the FL response was attributable to a cyclization-induced emission (CIE) that resulted from complex formation with L-tartarate (see figure).
The team tested the FL response of TPE-Zn toward the biologically relevant dicarboxylates. A practical level of FL increase was observed for a series of dicarboxylates. However, virtually no FL increase occurred in the presence of acetate, the reference monocarboxylate. This shows that TPE-Zn is able to differentiate dicarboxylates from monocarboxylates.
The relative FL intensity increased with decreasing spacer alkyl chain length except for malonate. This tendency is consistent with the proposed CIE mechanism: shorter alkyl chains enhance the degree of conformational restriction, leading to the higher FL intensity.
- Cyclization-Induced Turn-On Fluorescence System Applicable to Dicarboxylate Sensing,
Takao Noguchi, Bappaditya Roy, Daisuke Yoshihara, Youichi Tsuchiya, Tatsuhiro Yamamoto, Seiji Shinkai,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201304031