Nonheme iron dioxygenases catalyze a range of important reactions in nature. For example, AlkB repair enzymes catalyze the demethylation of alkylated DNA bases in humans, a vital reaction in the body that heals externally damaged DNA bases. However, there are many unanswered questions related to the activity of nonheme iron dioxygenases and the catalytic transformation of substrates.
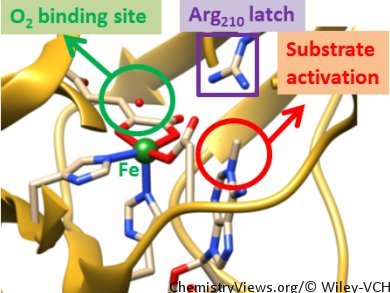
Sam P. de Visser, University of Manchester, UK, and colleagues have performed a quantum mechanics/molecular mechanics (QQ/MM) study on the demethylation of the N1-methyladenine fragment by AlkB repair enzymes. They identified only one feasible oxygen-binding site in the enzyme. This site was on the iron center trans to His131. The iron(III)–superoxo species is separated from the substrate by a considerable distance and its approach is blocked by an Arg residue. This prevents the iron(III)–superoxo from reacting with substrate.
However, the team discovered that the catalytically active iron(IV)–oxo intermediate can undergo isomerization assisted by the Arg residue in the substrate binding pocket. This isomerization brings the oxo group in close contact with the methyl group of the alkylated DNA base. This enables a subsequent rate-determining hydrogen-atom abstraction to form a radical intermediate followed by rebinding to give alcohol products.
These findings show that it is essential to separate the substrate- and oxygen-binding channels, otherwise the iron(III)–superoxo will react with substrate and prevent the repair reaction of the DNA.
- Quantum Mechanics/Molecular Mechanics Study on the Oxygen Binding and Substrate Hydroxylation Step in AlkB Repair Enzymes,
Matthew G. Quesne, Reza Latifi, Luis E. Gonzalez-Ovalle, Devesh Kumar, Sam P. de Visser,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303282