Iridium-catalyzed hydrogenation is a highly efficient method of reducing π-bonds in high selectivity and yields. Finding the best ligand is the key to obtaining full conversion and high enantiomeric excess in this reduction.
Pher Andersson and co-workers, University of Uppsala, Sweden, have shown that 2-substituted pyridines are efficiently and selectively dearomatized by using hydrogen gas and chiral analogues of Crabtree’s catalyst to generate chiral piperidines without side reactions:

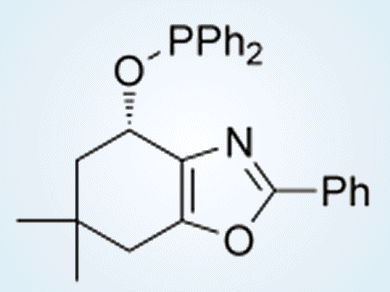
The choice of an oxazole-containing N,P-ligand (see picture), as well as it’s P-substitution was based on screening and tailoring to fit the electronic and steric properties of the substrates.
Pyridines are notoriously stable because of their aromaticity, but in this case the aromaticity was disrupted by forming N-iminopyridium ylides and also the use of iodine (rather than other halogens) as an additive. It is hoped that this method can be used for the synthesis of chiral piperidine-based building blocks.
- Iridium-Catalyzed Asymmetric Hydrogenation of Substituted Pyridines,
Alban Cadu, Puspesh K. Upadhyay, Pher G. Andersson,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300160