Lanthanide-based species have displayed excellent magnetocaloric effects, that is, on demagnetization the lattice entropy decreases resulting in an increase in magnetic entropy. This results in a cooling of the magnetic system and ultralow temperatures can be obtained. Coordination complexes have thus attracted increasing interest for magnetic refrigeration and are regarded as an efficient alternative to He, which is in short supply.
Eliseo Ruiz, Universitat de Barcelona, Spain, Ming-Liang Tong, Sun Yat-Sen University, China, and colleagues have made a series of heterometallic [LnIIIxCuIIy] complexes (Ln = Gd, Dy; see structures). They were synthesized at room temperature by a one-pot route involving three kinds of carbonyl-related reactions: cannizzaro reaction, aldol reaction, and oxidation. The latter two are addition reactions between homomolecules, with the organic ligand attacking itself rather than the usual route in which a small/solvent molecule acts as the nucleophile.
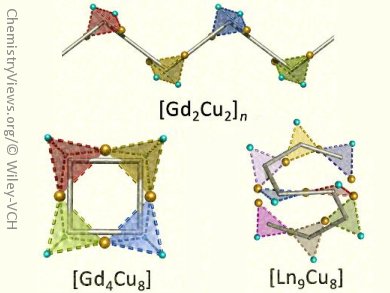
The resulting complexes displayed a range of molecular topologies, such as infinite zigzag [Gd2Cu2]n chain, square [Gd4Cu8] wheel, and S-shape [Ln9Cu8] (see picture), which consist of tetrahedral {Gd2Cu2} units or triangular {Ln2Cu} units.
[Dy9Cu8] exhibits single-molecule magnetic behavior and [GdxCuy] shows significant magnetocaloric effects.
This strategy, therefore, provides a promising route towards molecule-based magnetic materials.
- CuII–GdIII Cryogenic Magnetic Refrigerants and Cu8Dy9 Single-Molecule Magnet Generated by In Situ Reactions of Picolinaldehyde and Acetylpyridine: Experimental and Theoretical Study,
Jun-Liang Liu, Wei-Quan Lin, Yan-Cong Chen, Silvia Gómez-Coca, Daniel Aravena, Eliseo Ruiz, Ji-Dong Leng, Ming-Liang Tong,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303275