Titanium tetraiodide has recently become of considerable importance because of its use in the production of metallic titanium in industry. However, it is also a very useful iodotitanation reagent in organic synthesis. When it is used for iodoaldol reactions, the stereoselective synthesis of multifunctionalized alkenes is feasible, and the vinyl iodide moieties of the iodoaldol products can be applied as substrates to the metal-catalyzed cross-coupling and Nozaki–Hiyama–Kishi reaction.
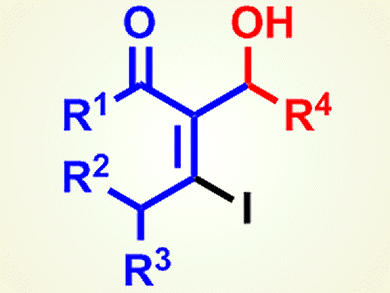
Makoto Shimizu and co-workers, Mie University, Japan, have developed a procedure that makes diastereoselective iodoaldol reaction of internal alkynyl ketones promoted by titanium tetraiodide possible. Such reactions have been previously limited to an intramolecular cyclization. The iodoaldol reaction of γ-alkoxy-α-β-alkynyl ketone derivatives proceeds smoothly to give vinyl iodides that have oxygen functional groups (pictured) with good diastereoselectivity. The iodoaldol products obtained can be transformed into tetrasubstituted furans by a palladium-catalyzed Sonogashira coupling and further cyclization.

- Diastereoselective Iodoaldol Reaction of γ-Alkoxy-α,β-Alkynyl Ketone Derivatives Promoted by Titanium Tetraiodide,
Iwao Hachiya, Shingo Ito, Shota Kayaki, Makoto Shimizu,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300165