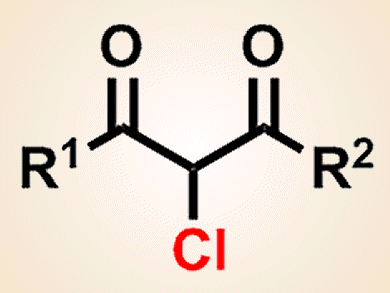
The combination of transition-metal catalysis and electrochemical oxidation provide environmentally benign synthetic methods. However, one of the challenges for these reactions is to avoid the use of excess amounts of relatively expensive supporting electrolytes.
Fumitoshi Kakiuchi and co-workers, Keio University, Japan, have developed a copper-catalyzed electrochemical chlorination of 1,3-dicarbonyl compounds by using inexpensive hydrochloric acid, which acts as both a chlorine source and a supporting electrolyte. Monochlorination products can be obtained selectively from various 1,3-dicarbonyl compounds and hydrochloric acid in the presence of a copper catalyst.

One of the appealing features of the catalytic electrochemical chlorination is that simple extraction removes all compounds not originating from the starting 1,3-dicarbonyl compounds. Therefore, the crude materials obtained by extraction can be directly used for further transformations, such as reduction to the corresponding β-hydroxy esters by sodium borohydride. The group is currently investigating the application of this technique to other transformations.
- Copper-Catalyzed Electrochemical Chlorination of 1,3-Dicarbonyl Compounds Using Hydrochloric Acid,
Kazuya Tsuchida, Takuya Kochi, Fumitoshi Kakiuchi,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300168