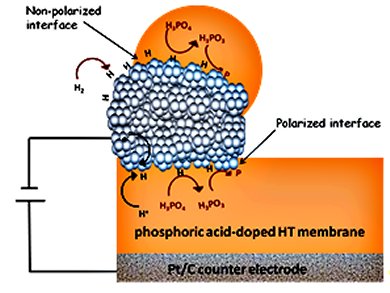
High-temperature proton-exchange membrane fuel cells (HT PEMFCs) have a higher carbon monoxide tolerance and better water management compared to low-temperature PEMFCs. Therefore, HT PEMFCs are attractive for stationary power supplies and hydrogen purification by operation as hydrogen pumps. These allow hydrogen to pass from the anode by oxidizing hydrogen to the cathode by reducing protons. However, HT PEMFCs operate under hot and dry conditions, restricting the available techniques for investigating their fundamental properties.
Elena Savinova and her co-workers in Strasbourg, France, Trieste, Italy, and Patras, Greece, have used scanning photoelectron microscopy (SPEM) for in situ monitoring of the morphology, surface composition, and chemical state of the electrode/electrolyte interface of a HT PEMFC based on a phosphoric-acid-imbibed polymer membrane.
A spectroscopic signature was obtained, confirming the long-anticipated formation of reduced phosphorus species. By exploring the spatial distribution of these reduced species it was concluded that the reduction process is Pt-catalyzed. By monitoring this Pt-catalyzed reduction of phosphoric acid with hydrogen, the long-disputed nature of the products can be assigned to phosphorous acid and elementary phosphorus.
 A Scanning Photoelectron Microscopy Study of the Pt/Phosphoric-Acid-Imbibed Membrane Interface under Polarization,
A Scanning Photoelectron Microscopy Study of the Pt/Phosphoric-Acid-Imbibed Membrane Interface under Polarization,
Won Doh, Luca Gregoratti, Matteo Amati, Spyridon Zafeiratos, Yeuk T. Law, Stylianos Neophytides, Alin Orfanidi, Maya Kiskinova, Elena R. Savinova,
ChemElectroChem 2013.
DOI: 10.1002/celc.201300134
ChemPubSoc Europe’s new journal ChemElectroChem is dedicated to covering the entire scope of pure and applied electrochemistry.