The syntheses of natural heterocyclic compounds and, in particular, indole alkaloids have been crucial drivers for the development of modern organic chemistry. Monoterpene indole alkaloids constitute a large and diverse class of natural products with all levels of molecular complexity and a wealth of biological activities. However, planning an appropriate, efficient, and atom-economic strategy for the total syntheses of these alkaloids is a challenging task, with the best strategies allowing access to a variety of different alkaloid structures with a minimum number of steps.
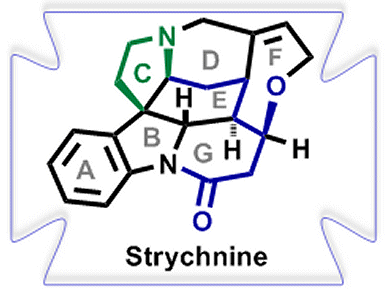
Hans-Ulrich Reissig and co-workers, Freie Universität Berlin, Germany, intrigued by the complex core structure of strychnine, have employed a samarium diiodide (SmI2)-induced cyclization-trapping method to easily construct highly functionalized dihydroindole derivatives.

These transformations led to the tetracyclic key building block suitable for the synthesis of the ABCEG rings of strychnine. An atom-economic synthesis was also developed employing a SmI2-induced cascade reaction of dimeric indolyl ketones to afford the ABGE core structure of strychnine in one step.
- Towards the Core Structure of Strychnos Alkaloids Using Samarium Diiodide-Induced Reactions of Indole Derivatives,
Christine Beemelmanns, Steffen Gross, Hans-Ulrich Reissig,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302795