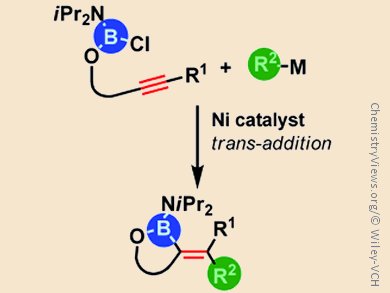
Exploration of new synthetic access to organoboronic acids is highly valuable because the demand for such compounds in organic synthesis, pharmaceutical sciences, and materials chemistry is sharply increasing. Catalytic carboboration, in which carbon and boron groups add concomitantly across carbon–carbon multiple bonds, is one such method of producing these compounds.
Michinori Suginome and colleagues, Kyoto University, Japan, describe the details of a nickel-catalyzed cyclization of chloro(diisopropylamino)boryl homopropargyl ethers in the presence of organostannane or organozirconium reagents as the sources of organic groups. The reaction is initiated by oxidative addition of the B–Cl bond to the nickel(0) species as confirmed by isolation of the corresponding organonickel intermediate. The following elementary steps, including cis/trans isomerization of an alkenylnickel chloride intermediate and subsequent transmetalation, lead to the formation of five-membered-ring carboboration products by trans-addition.
This process along with previously reported palladium-catalyzed carboborations allows efficient synthesis of new functionalized organoboronic acids derivatives that are otherwise difficult to synthesize.
- Nickel-Catalyzed Cyclizative trans-Carboboration of Alkynes through Activation of Boron–Chlorine Bonds by Using Organometallic Reagents as Donors of Organic Groups,
Masaki Daini, Akihiko Yamamoto, Michinori Suginome,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300164