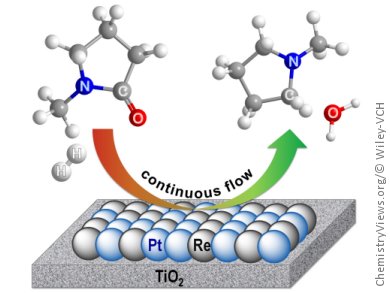
Flow catalysis is combined with amide hydrogenation to produce amines selectively in up to 100 % yields. Amine hydrogenation was previously limited to C–N bond cleavage or monohydrogenation, yielding a mixture of amine and alcohol or hemiaminals, respectively. That was, until David John Cole-Hamilton, University of St. Andrews, UK, and co-workers developed a homogeneous ruthenium catalyst to selectively produce amines from amides.
Cole-Hamilton and co-workers, building on subsequent developments in heterogeneous catalysis, have now developed a continuous flow system for the hydrogenation of amides to amines over a titania-supported platinum–rhenium catalyst. The high-throughput upward-flow vertical reactor can stream liquids and gases simultaneously through the packed bed containing the air-stable catalyst 4 % Pt–4 % Re/TiO2. The catalyst- and impurity-free products are then collected directly in the solvent, with a metal contamination below the detection limit of 1 ppm.
This process, with its easy separation and extremely low contamination levels, has potential for application even in late-stage industrial pharmaceutical production. Substrates tested include N-methylpyrrolidone and N-methylpropanamide. The next challenge is to extend the substrate scope by developing a heterogeneous catalyst that also circumvents aromatic-ring hydrogenation.
- The First Continuous Flow Hydrogenation of Amides to Amines,
Jacorien Coetzee, Haresh G. Manyar, Christopher Hardacre, David J. Cole-Hamilton,
ChemCatChem 2013, 5, 2843–2847.
DOI: 10.1002/cctc.201300431