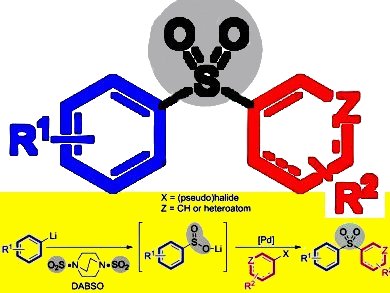
Aryl, heteroaryl, and alkenyl sulfones are versatile intermediates in organic synthesis, of particular pharmaceutical relevance, and exhibit an extensive and broad range of biological activities. In addition, sulfone-containing polymers display novel properties as materials. A variety of methods are known for the preparation of sulfones, but the majority of these feature associated limitations.
Michael C. Willis, University of Oxford, UK, and colleagues have demonstrated the synthesis of a broad range of aryl, heteroaryl, and alkenyl sulfones through a convergent three-component palladium-catalyzed coupling approach. Their method allows the straightforward production of varied sulfones through the union of two readily available coupling partners: an aryl lithium species and aryl, heteroaryl, or alkenyl (pseudo)halides; with SO2 provided by the easy-to-handle bench-stable solid surrogate DABSO (pictured). The use of an electron-poor XantPhos-type ligand offers much improved yields over XantPhos (4,5-bis(diphenylphosphino)-9,9-dimethylxanthene) itself. In addition, the team introduced ortho functionality by exploiting the ability of the sulfone group to direct ortho metalation.
The researchers demonstrated the utility of the methodology through the synthesis of a medicinal agent that is currently in development at AstraZeneca. They utilized a versatile bis-halogenated quinolone as the starting material.
Evidence for the nontrivial nature of the preparation of aryl–aryl and related sulfones was found in the observation that of the 36 sulfone products more than 90 % are novel compounds.
- Palladium-Catalyzed Three-Component Diaryl Sulfone Synthesis Exploiting the Sulfur Dioxide Surrogate DABSO,
Edward J. Emmett, Barry R. Hayter, Michael C. Willis,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201305369