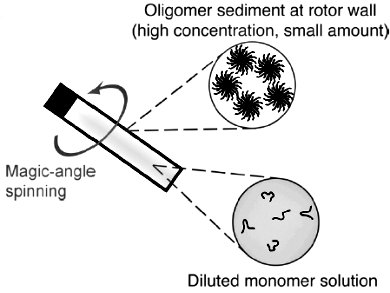
β-Amyloid (Aβ) is the main component of certain deposits found in the brains of patients with Alzheimer’s disease.
Claudio Luchinat, Magnetic Resonance Center (CERM), University of Florence, Italy, and colleagues have shown that termed sedimented solutes NMR (SedNMR) allows for collecting and trapping of AβM40 aggregates in a fully hydrated environment without adding cosolvents or interaction partners. Therefore, this provides a unique way to access the formation kinetics and structural features of these species with reduced perturbations.
To immobilize biomolecules and make them amenable for SedNMR studies, they have introduced sedimentation through ultracentrifugation, either by magic angle spinning (in situ) or preparative ultracentrifuge (ex situ). In situ SedNMR was used to address the kinetics of formation of soluble Aβ assemblies by monitoring the disappearance of the monomer and the appearance of the oligomers simultaneously. Ex situ SedNMR allows to select different oligomeric species and to reveal atomic-level structural features of soluble Aβ assemblies.
According to the team this approach can be easily applied to other amyloid systems and, more generally, to the study of many supramolecular assembly processes.
- Formation Kinetics and Structural Features of β-Amyloid Aggregates by Sedimented Solute NMR,
Ivano Bertini Gianluca Gallo, Magdalena Korsak, Claudio Luchinat, Jiafei Mao, Enrico Ravera,
ChemBioChem 2013, 14, 1891–1897.
DOI: 10.1002/cbic.201300141
Issue 14/2013 of ChemBioChem is dedicated to the memory of the noted Italian scientist Ivano Bertini, who died in July 2012.
Editorial:
 Magnetic Resonance Spectroscopy in Bio(in)organic Chemistry and in Mechanistic Systems Biology: A Tribute to Ivano Bertini,
Magnetic Resonance Spectroscopy in Bio(in)organic Chemistry and in Mechanistic Systems Biology: A Tribute to Ivano Bertini,
Harald Schwalbe, Joshua Telser
ChemBioChem 2013, 14, 1671–1675.
DOI: 10.1002/cbic.201300451