Tin(II) chloride reacts with transition-metal complexes bearing chloride ligands to give the corresponding transition metal/tin complexes, in which the tin enhances the capacity of the transition metals for catalysis, such as for hydrogenation, polymerization, hydroformylation, and coupling reactions. The labilization of ligands in the transition-metal complex to tin ligands and/or the dissociation of tin ligands themselves on the transition metal/tin complexes is the source of the catalysis.
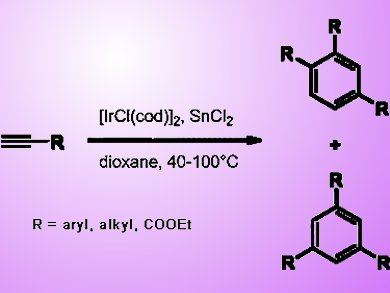
Yoshiro Masuyama and co-workers from Sophia University, Tokyo, Japan, discovered that tin(II) chloride induces iridium(I)-catalyzed cyclotrimerization of terminal alkynes to prepare trisubstituted benzenes. An iridium/tin complex, which is prepared from [IrCl(cod)]2 and tin(II) chloride, seems to be the active catalytic species, although it has not yet been possible to isolate and characterize such a complex. As tin(II) chloride and [IrCl(cod)]2 are tractable, the cyclotrimerization can be carried out with ease.
The group hopes to develop new types of iridium/tin-catalyzed reactions and apply tin(II) chloride to the activation of other transition-metal complexes for developing new catalytic reactions.
 Cyclotrimerization of Terminal Alkynes Catalyzed by a Phosphine-Free Chloro(1,5-cyclooctadiene)iridium(I) Dimer and Induced by Tin(II) Chloride,
Cyclotrimerization of Terminal Alkynes Catalyzed by a Phosphine-Free Chloro(1,5-cyclooctadiene)iridium(I) Dimer and Induced by Tin(II) Chloride,
Yoshiro Masuyama, Kana Miyazaki, Noriyuki Suzuki,
Asian J. Org. Chem. 2013, 2, 750–754.
DOI: 10.1002/ajoc.201300132