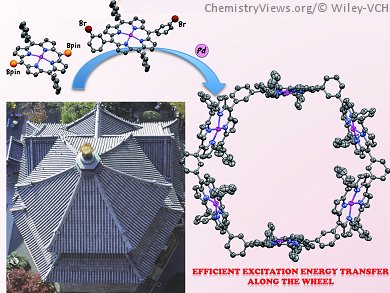
Bacteriochlorophyll pigments that are arranged in wheel-like architectures are found in light-harvesting complexes, such as LH2 and LH1. These wheel-like structures facilitate energy migration to the reaction center. In artificial photosynthesis studies, particular attention is therefore being given to construction of covalently linked cyclic porphyrin arrays, in which the manipulation of the inter-porphyrinic interaction is essential to achieve efficient excitation energy transfer.
Atsuhiro Osuka, Kyoto University, Japan, and colleagues have developed Ag(I)-promoted meso–meso coupling reactions of 5,15-diaryl-substituted Zn(II)-porphyrins as a means to covalently connect porphyrins. By using a Suzuki–Miyaura coupling reaction through a one-pot or a stepwise route, the team synthesized a cyclic dodecameric porphyrin oligomer, in which six meso–meso-linked zinc(II) diporphyrin subunits are bridged by 1,3-phenylene spacers to form a large hexagonal ring. This synthetic strategy was extended to a 1,3-phenylene-bridged meso–meso-linked diporphyrin dimer, which led to production of a larger hexagonal ring consisting of 24 porphyrins.
Both porphyrin wheels display efficient excitation energy hopping (EEH) along the wheel; EEH rates have been estimated to be 4 and 35 ps for the 12-meric and 24-meric wheels, respectively. In addition, the porphyrin hexamer is interesting as a shape-persistent organic molecule.
- A 1,3-Phenylene-Bridged Hexameric Porphyrin Wheel and Efficient Excitation Energy Transfer along the Wheel,
Hua-Wei Jiang, Sujin Ham, Naoki Aratani, Dongho Kim, Atsuhiro Osuka,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302361