Asymmetric synthesis of all-carbon quaternary stereogenic centers presents a significant challenge in organic synthesis and has attracted much interest over the last decade. One of the many synthetic solutions disclosed in the literature are rhodium and copper-catalyzed asymmetric conjugate additions (ACAs) using organometallic reagents. However, despite their synthetic potential, only a few metal-catalyzed ACA reactions have been reported for alkenyl nucleophiles to generate quaternary stereogenic centers.
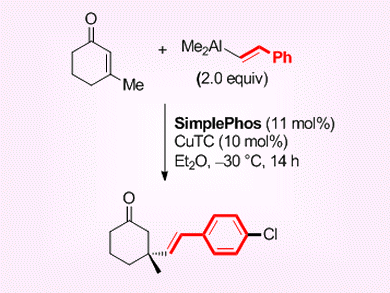
Alexandre Alexakis and Daniel Müller, University of Geneva, Switzerland, have described the asymmetric copper-catalyzed conjugate addition of alkenylaluminum reagents to 3-substituted cyclic enones to form stereogenic all-carbon quaternary centers.
.jpg)
A large number of alkenyl nucleophiles can undergo the copper-catalyzed ACA with good to very good levels of enantioselectivity, typically 70–90 % ee, when cyclohexenones and derivatives are used as the substrate.
The reaction protocols are practical and the ligand and copper salt used are relatively inexpensive.
- Formation of Quaternary Stereogenic Centers by Copper-Catalyzed Asymmetric Conjugate, Addition Reactions of Alkenylaluminums to Trisubstituted Enones,
Daniel Müller, Alexandre Alexakis,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302856