Conjugate addition reactions play a crucial role in organic synthesis. Research on these transformations has been boosted by the wide diversity of compounds that can serve as nucleophiles and electrophiles in the reaction to generate a varied array of products. In contrast to carbonyl substrates and nitroalkenes, asymmetric conjugate addition to α,β-unsaturated imines has been scarcely explored, probably due to the lower electrophilicity of these substrates. Only a small number of examples regarding the enantioselective 1,4-nucleophilic addition of carbon nucleophiles to unsaturated imines have been reported.
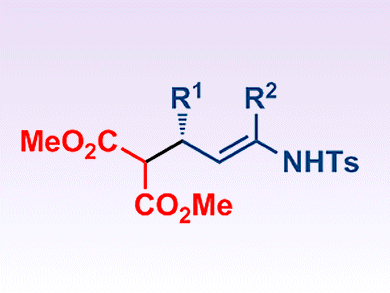
Gonzalo Blay, José R. Pedro and co-workers, Universitat de València, Spain, report the first example of the asymmetric conjugate addition of malonate esters to α,β-unsaturated N-sulfonyl imines. The reactions are catalyzed by PyBOX/La(OTf)3 complexes in the presence of 4 Å molecular sieves. They give the corresponding E enamines bearing a stereogenic center at the allylic position with good yields and enantiomeric ratios up to 97:3 (see scheme).
.gif)
These compounds are effective synthons for the preparation of optically active δ-aminoesters bearing two stereogenic centers at the β and δ-positions, and for the preparation of optically active piperidones.
- Asymmetric Conjugate Addition of Malonate Esters to α,β-Unsaturated N-Sulfonyl Imines: An Expeditious Route to Chiral δ-Aminoesters and Piperidones,
Miguel Espinosa, Gonzalo Blay, Luz Cardona, José R. Pedro,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302687

![Diazine-Tetraphenylethylene Cyclo[6]arenes for Molecular Recognition in Solution and Aggregate States](https://www.chemistryviews.org/wp-content/uploads/2025/11/ChemistryViews-2.70171-125x94.png)
