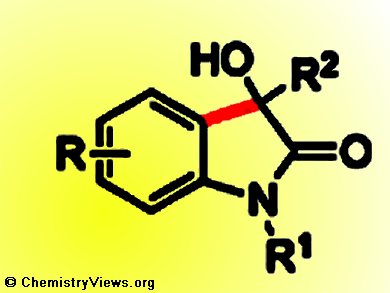
3-Hydroxy-2-oxindoles are important motifs in natural products and pharmaceutical molecules. Transition-metal-catalyzed direct C–H addition of electron-rich aromatic and heteroaromatic compounds to carbonyl compounds provides a straightforward and more sustainable route to construct them.
Shang-Dong Yang, Hong-Li Wang, and co-workers, Lanzhou University, China, have developed a scandium triflate (Sc(OTf)3) catalyzed intramolecular cyclization to synthesize 3-hydroxy-2-oxindoles by direct C–H bond addition to ketones.
.jpg)
In contrast to the traditional methods of nucleophilic conjugate additions of organometallic reagents, the addition of electron-rich reagents to isatins, and intramolecular addition of aryl or vinyl chlorides to ketoamides, this new protocol is simple, environmentally benign, and omits the complicated preparation of organometallic reagents and halogenation procedures.
Future studies on the system will include further applications in intramolecular addition to ketones.
- Scandium(III) Triflate Catalyzed Direct Cyclization of Ketoamides for the Synthesis of 3-Hydroxy-2-Oxindoles,
Hong-Li Wang, Ya-Min Li, Gang-Wei Wang, Heng Zhang, Shang-Dong Yang,
Asian J. Org. Chem. 2013, 2, 486–490.
DOI: 10.1002/ajoc.201300057